Draw a Diagram to Show How Kcl Dissolves in Water
11.ii: Electrolytes
- Page ID
- 38250
- Define and requite examples of electrolytes
- Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes
- Relate electrolyte strength to solute-solvent bonny forces
When some substances are dissolved in water, they undergo either a physical or a chemical alter that yields ions in solution. These substances institute an of import class of compounds called electrolytes. Substances that practise not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known every bit a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may exist identified equally potent, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To comport electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metal wires, in which example the mobile, charged entities are electrons. Solutions may also carry electricity if they contain dissolved ions, with conductivity increasing every bit ion concentration increases. Applying a voltage to electrodes immersed in a solution permits cess of the relative concentration of dissolved ions, either quantitatively, by measuring the electric current flow, or qualitatively, by observing the brightness of a lite bulb included in the circuit (Effigy \(\PageIndex{1}\)).
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in Figure \(\PageIndex{2}\). The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an of import function in the dissolution of ionic compounds in water.
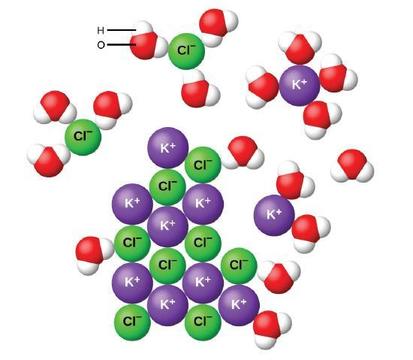
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces betwixt them. This process represents a physical change known every bit dissociation. Under most conditions, ionic compounds will dissociate about completely when dissolved, and so they are classified as potent electrolytes.
Let us consider what happens at the microscopic level when we add together solid KCl to water. Ion-dipole forces concenter the positive (hydrogen) stop of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The h2o molecules penetrate between individual K+ and Cl− ions and surroundings them, reducing the stiff interionic forces that bind the ions together and letting them move off into solution as solvated ions, as Figure shows. The reduction of the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution, resulting in an increment in the disorder of the system every bit the ions change from their fixed and ordered positions in the crystal to mobile and much more matted states in solution. This increased disorder is responsible for the dissolution of many ionic compounds, including KCl, which dissolve with absorption of heat.
In other cases, the electrostatic attractions between the ions in a crystal are so big, or the ion-dipole attractive forces between the ions and h2o molecules are and so weak, that the increase in disorder cannot recoup for the energy required to separate the ions, and the crystal is insoluble. Such is the case for compounds such as calcium carbonate (limestone), calcium phosphate (the inorganic component of os), and atomic number 26 oxide (rust).
Why do Ionic Solids Dissolve in Water (Ion-Dipole Imf)?: https://youtu.exist/yz1Ml0Q8b_I
Covalent Electrolytes
Pure water is an extremely poor usher of electricity because it is just very slightly ionized—only about ii out of every 1 billion molecules ionize at 25 °C. Water ionizes when ane molecule of h2o gives upward a proton to some other molecule of h2o, yielding hydronium and hydroxide ions.
\[\ce{H_2O (l)+ H_2O (l) \rightleftharpoons H_3O^{+} (aq) + OH^{−} (aq)} \label{11.3.ii}\]
In some cases, we observe that solutions prepared from covalent compounds comport electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalent HCl molecules. This gas contains no ions. Still, when we dissolve hydrogen chloride in h2o, we find that the solution is a very good conductor. The water molecules play an essential part in forming ions: Solutions of hydrogen chloride in many other solvents, such as benzene, practice not conduct electricity and exercise not contain ions.
Hydrogen chloride is an acid, and and so its molecules react with water, transferring H+ ions to form hydronium ions (\(H_3O^+\)) and chloride ions (Cl−):
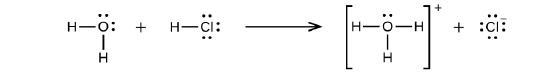
This reaction is essentially 100% complete for HCl (i.due east., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that but react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of this text on reaction classes and stoichiometry.
Summary
Substances that dissolve in water to yield ions are chosen electrolytes. Electrolytes may exist covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Dissolution of an ionic chemical compound is facilitated by ion-dipole attractions between the ions of the compound and the polar water molecules. Soluble ionic substances and strong acids ionize completely and are strong electrolytes, while weak acids and bases ionize to just a minor extent and are weak electrolytes. Nonelectrolytes are substances that do not produce ions when dissolved in h2o.
Glossary
- dissociation
- concrete process accompanying the dissolution of an ionic chemical compound in which the compound's constituent ions are solvated and dispersed throughout the solution
- electrolyte
- substance that produces ions when dissolved in water
- ion-dipole attraction
- electrostatic allure between an ion and a polar molecule
- nonelectrolyte
- substance that does not produce ions when dissolved in water
- strong electrolyte
- substance that dissociates or ionizes completely when dissolved in h2o
- weak electrolyte
- substance that ionizes merely partially when dissolved in water
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_(OpenSTAX)/11%3A_Solutions_and_Colloids/11.2%3A_Electrolytes
Belum ada Komentar untuk "Draw a Diagram to Show How Kcl Dissolves in Water"
Posting Komentar